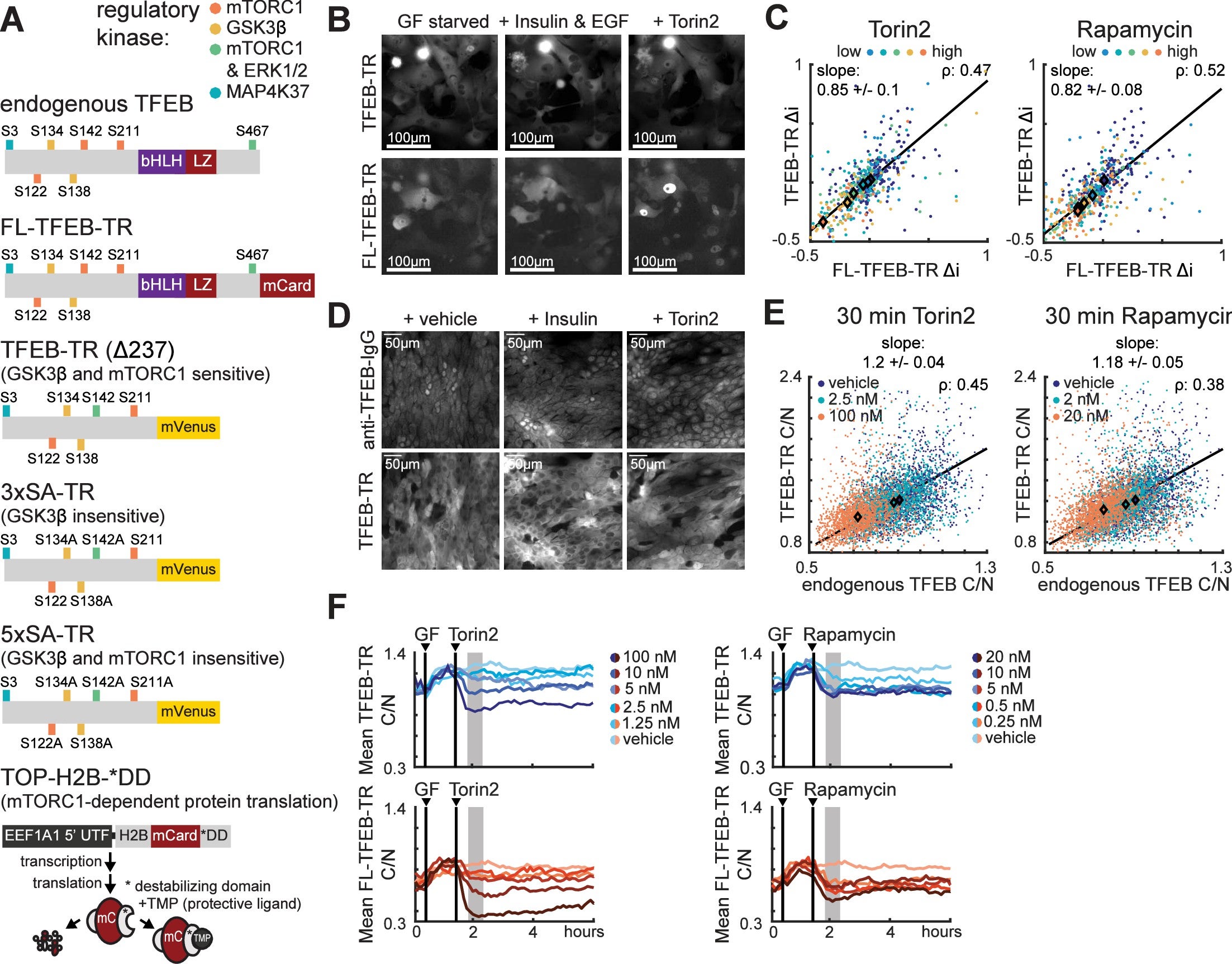
Continuous sensing of nutrients and growth factors by the mTORC1-TFEB axis
Breanne Sparta, Nont Kosaisawe, Michael Pargett, Madhura Patankar, Nicholaus DeCuzzi, John G Albeck
What’s mTORC1’s role in metabolism?
mTORC1 is a central protein for regulating cell homeostasis and senses the presence of growth hormones. Despite being a keystone kinase in homeostasis, the individual cell response over time is not well quantified. Previous methods for studying mTORC1 were population averaging methods (such as immunoblots) or fixed single-cell snapshots (such as immunofluorescence) that obscured the heterogeneous activity of single cells. In this paper, MCF10a cells were imaged with fluorescently-tagged TFEB (a target of mTORC1) to confirm that mTORC1-TFEB signaling responds continuously to individual, sequential, or simultaneous treatment with amino acids and the growth factor insulin.
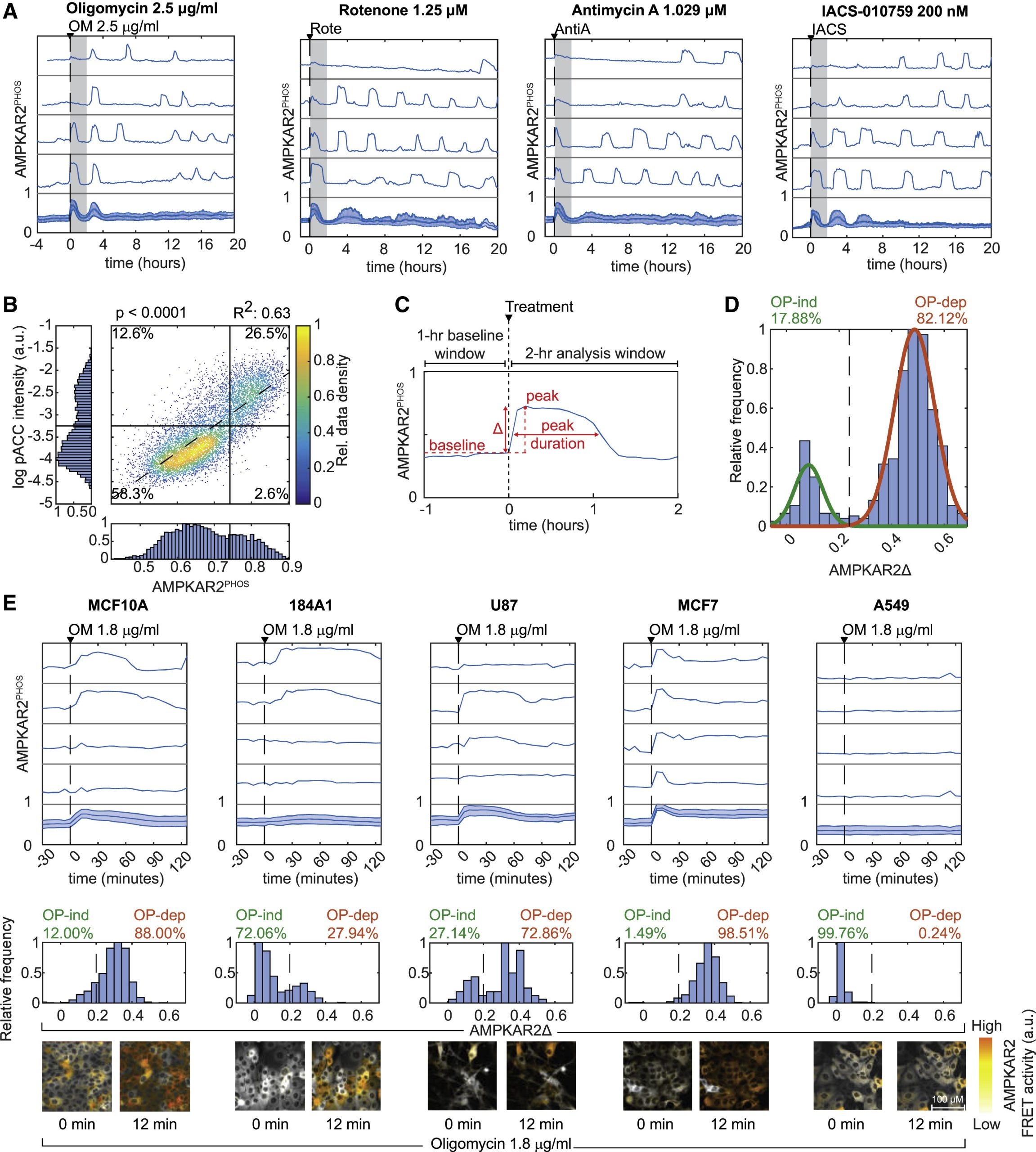
Transient phases of OXPHOS inhibitor resistance reveal underlying metabolic heterogeneity in single cells
Nont Kosaisawe, Breanne Sparta, Michael Pargett, Carolyn K. Teragawa, John G. Albeck
Do genetically identical cells have different types of metabolism?
The influence on the heterogeneity of cells between metabolic states plays an unknown role in physiology and pharmacology. In this paper, cells with fluorescent reporters for ATP, ADP/ATP ratio, or the activity AMPK (an energy-sensing kinase) across several cell types are studied. The results show that some cells in a clonal population will enter a state resistant to activation of AMPK and reduction of ADP/ATP ratio. Resistance is linked to a multi-factorial combination of increased glucose uptake, reduced protein biosynthesis, and G0/ G1 cell-cycle status. Our results reveal dynamic fluctuations in cellular energetic balance and provide a basis for measuring and predicting the distribution of cellular responses to OXPHOS inhibition.
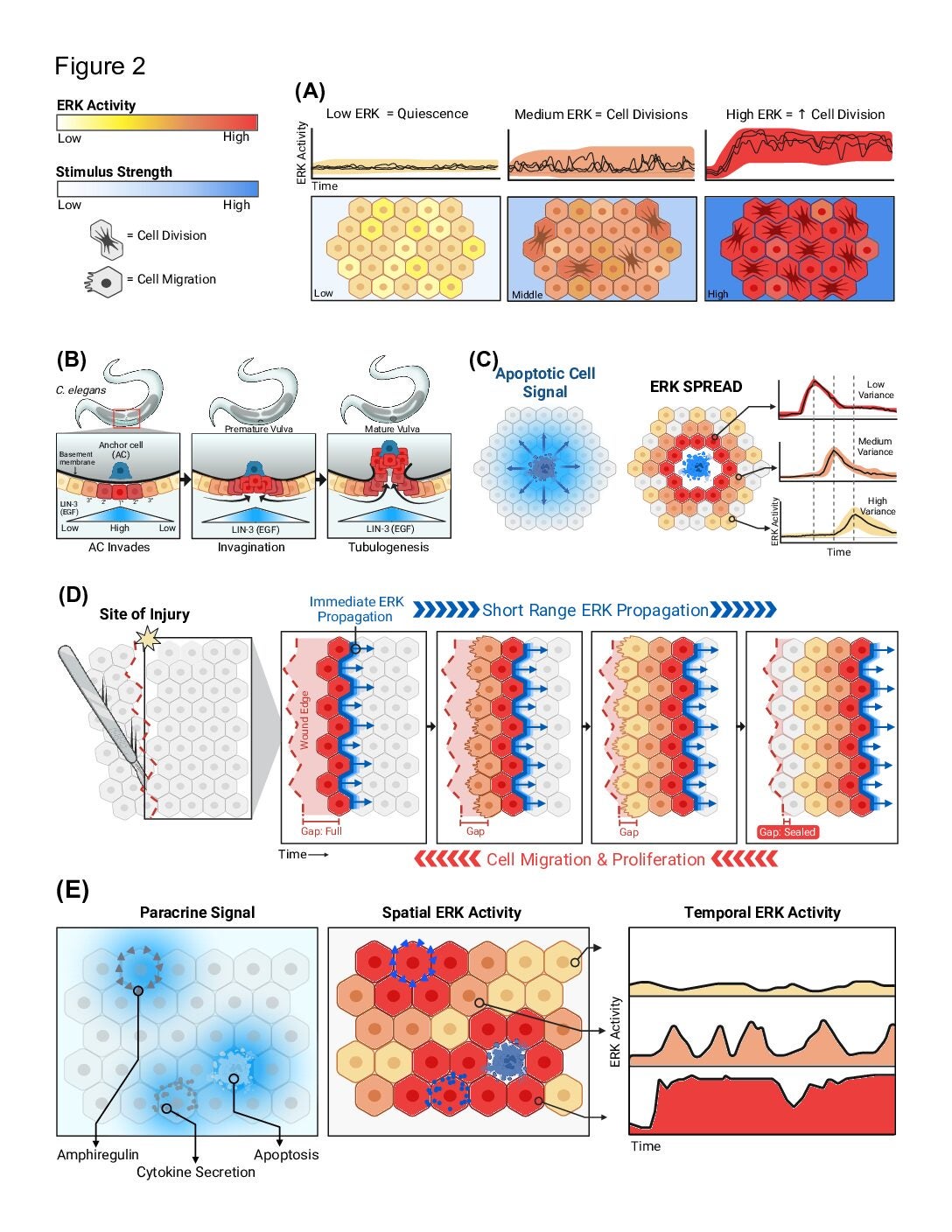
Systems-Level Properties of EGFR-RAS-ERK Signaling Amplify Local Signals to Generate Dynamic Gene Expression Heterogeneity
Alexander E. Davis, Michael Pargett, Setafn Siebert, Taryn Gillies, Yangin Choi, Svannah J. Tobin, Abhineet R. Ram, Vaibhav Murthy, Celina Juliano, Gerald Quan, Mina J. Bissell, John G. Albeck
How does cancer use AREG to modulate ERK activity?
A tumor may initially appear to be simply “one” kind of cancer throughout, but the composition of tumors is variable. Intra-tumoral heterogeneity is associated with aggressive tumor behavior, therapy resistance, and poor patient outcomes. Essentially this means that tumors have the capacity to adapt leading to metastasis and drug-resistance. Here in this study, a live-cell fluorescent reporter model was developed to study the moment-to-moment variation in ERK activity in tumor cells and the variation in gene expression. Analysis shows that the microenvironment created from paracrine signaling is enough to drive differential gene expression between neighboring cells.
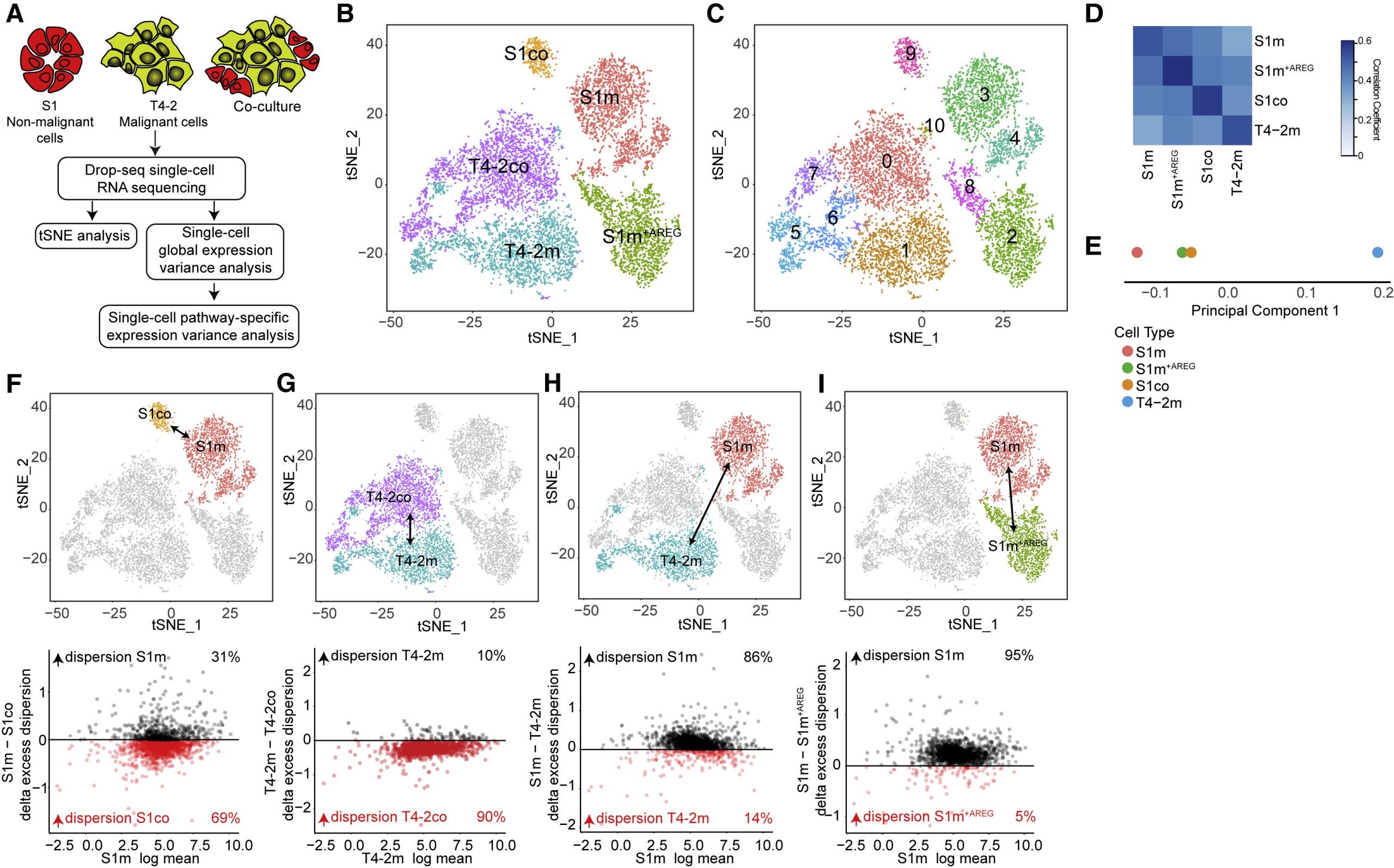
Oncogenic mutant RAS signaling activity is rescaled by the ERK/MAPK pathway
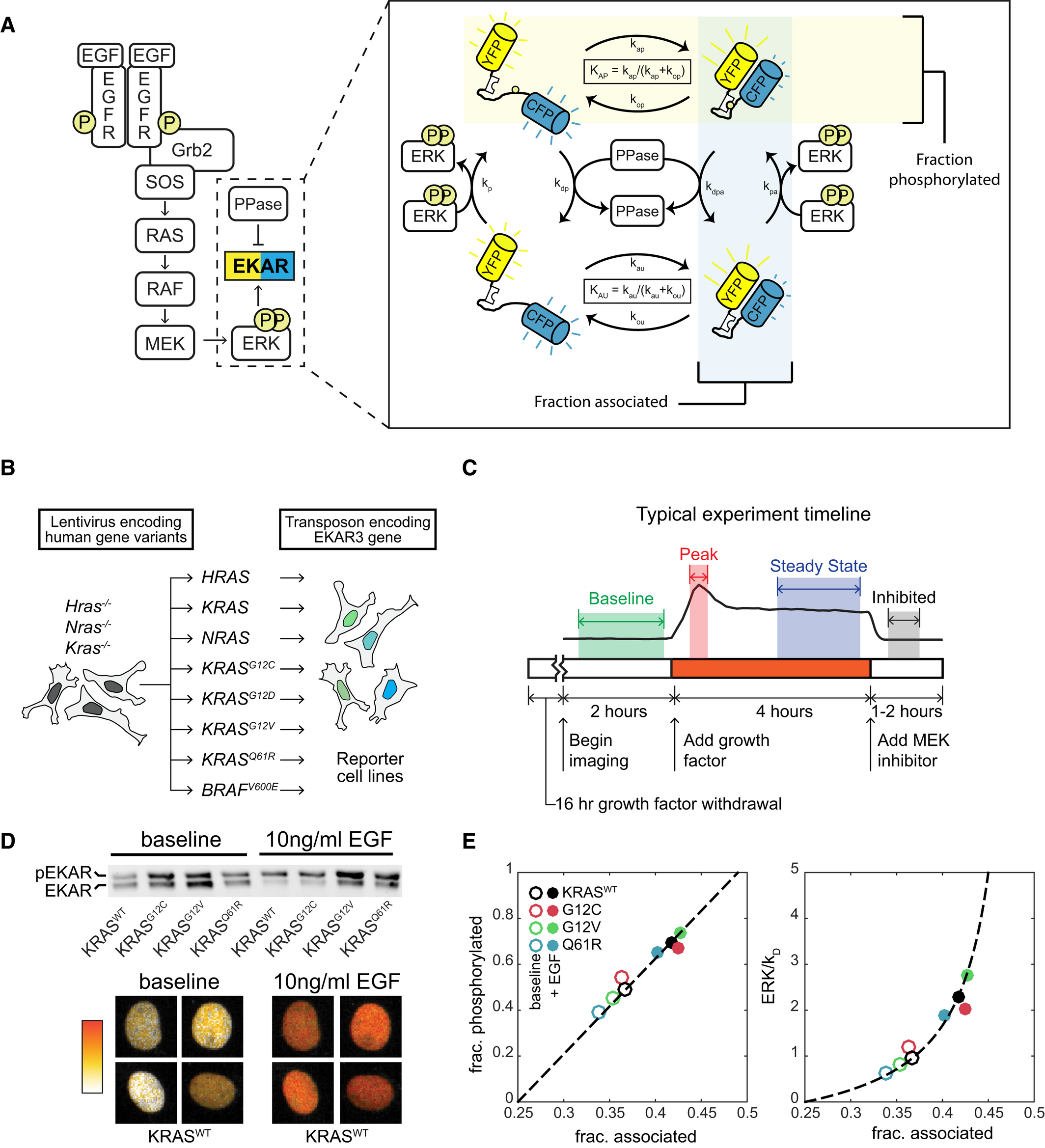
Taryn E Gillies, Michael Pargett, Jillian M Silva, Carolyn K Teragawa, Frank McCormick, and John G Albeck
What is RAS doing in cancer?
RAS mutations are common in many types of cancer. RAS GTPases act as molecular switches that will alternate between two states: inactive with GDP bound and active with GTP bound. When active, the RAS GTPase can drive the expression of growth genes. Guanidine Exchange Factor (GEFs) activates RAS by dissociating the GDP to return RAS to its active GTP-bound state. GTPase Activating Proteins (GAPs) inactivate RAS by increasing its GTPase activity. The net behavior of RAS is determined by the ratio of GAPs and GEFs to ultimately yield a binary-like mode of operation. In this paper, researchers used live cell experiments to study KRAS mutations on downstream ERK activity in new levels of resolution.
A guide to ERK dynamics, part 1: mechanisms and models
This is part one of two comprehensive reviews of the mechanism and techniques to measure the wildly variable nature of Extracellular Response Kinase (ERK). ERK is a driver of cell growth, but when dysregulated it becomes a source of disease and cancer. In recent history, ERK studies have been utilizing optogenetic and live-cell readouts to gather more data resolution on the moment-to-moment behavior of ERK and this paper explains the methods, mathematical models, and future directions associated with ERK research.

A guide to ERK dynamics, part 2: downstream decoding
Why does the pattern of ERK activity matter?
This is part two of two in the comprehensive reviews of ERK. In this part, the paper discusses the heterogeneous behavior of ERK activity and how the advent of live cell data has shown researchers ERK’s context dependent nature. This paper will summarize the four major functional modes of ERK signaling: cell population size, gradient-based patterning, wave propagation of morphological changes, and gene expression states.